Experimenting with Secondary Care Medicines Data
Posted in News, Publications
by Jason Pruchniewicz
At Rx-info we have always believed that data is crucial to the efficient and effective use of medicines.
The company was set up because our founder Colin Richman was frustrated at the lack of quickly accessible data while he was working as an NHS pharmacist.
Today, we provide a system that removes the complex nature of working with large quantities of non-standard data so answers can be found quickly.
For the past year Rx-info have been working with NHSBSA to supply a standardised dataset containing secondary care medicines data (SCMD) which is freely available for public use, and we have created the following reports to demonstrate what it could it could be used for.
The values within the SCMD are based on the dm+d (dictionary of medicines and devices) and show the unit and the quantity which could be, for example, the total number of capsules, tablets, or mls issued in a month. A stumbling point with this is that outside of a single Virtual Medicinal Product the values are not comparative and therefore may not provide accurate reporting.
In order to allow us to report more accurately and efficiently we have used additional reference material from the World Health Organization Collaborative Centre and applied an Anatomical Therapeutic Chemical classification (ATC) and a Defined Daily Dose (DDD). See the footnote for more information on this.
All the reports here contain data from the most recently available timeframe of January 2019 – June 2020.
Systemic Antibacterial Medicines Monitoring Examples
Users of the data can now monitor the use of antimicrobials to help improve antimicrobial stewardship which remains one of the most important national and indeed global public health challenges.
Using the raw Virtual Medicinal Product (VMP) quantity values from the Secondary Care dataset we have produced the following report:
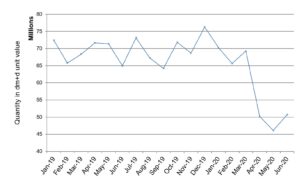
We have used the ATC classification mentioned above to group all systemic antibacterial VMPs in the J01 category as defined by the WHO within the Secondary Care dataset into one report. The quantity value is as defined in the Secondary Care dataset additional information. The results are shown on a month-by-month basis.
Using DDD values for accurate reporting
Using the methodology described in the footnotes we have assigned a DDD value to each unit of measure for each virtual medicinal product to allow us to directly compare medicines in different forms. The result gives a similar result, yet the methodology is more robust.
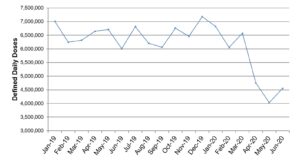
It can be seen in both reports that a significant drop in antimicrobial issuing occurred at the start of the Covid-19 pandemic.
Further to the standardisation of the medicinal forms we have used data from the NHS Monthly Activity Returns dataset to show the number of admissions that occur within Secondary Care trusts. The total admissions for the time period was used as a denominator against the total DDD of the data. For the purposes of this report we have decided to total the values labelled ‘Elective G&A Admissions’, ‘Elective G&A Daycase admissions’ and ‘Total non-elective G&A admissions’ and use this to calculate the number of DDDs used per 1,000 admissions.
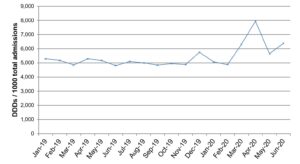
This gives an interesting picture suggesting that whilst overall antimicrobial use decreased during the pandemic, when compared to the number of patients admitted, the use has increased. This would coincide with an increased number of critical patients requiring more intensive treatment overall including antimicrobial use.
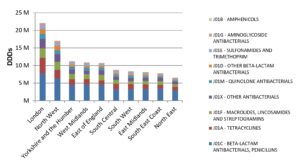
The Secondary Care dataset has Trust Organisation Data Service codes assigned to the data which allows reports to be created that show data from individual trusts.
Here is a similar report showing the number of DDDs used within each antibacterial type separated by geographical region:
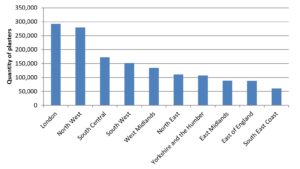
Lidocaine 5% medicated plaster issuing
The Secondary Care dataset also allows quick analysis of individual products such as in this example of lidocaine 5% medicated plasters which have limited beneficial evidence. Trusts could benefit from a cost saving by ensuring prescribing is appropriate.
Actual cost data is only available to the NHS and its users. A publicly available ‘Net Ingredient Cost’ value could be used, but is likely to be different to the actual cost.
Inhalers and their environmental impact
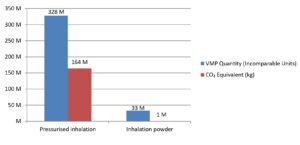
Metered dose inhalers use a propellant to deliver medicine to a patient’s respiratory system, but these are often hydrofluorocarbons which are harmful to the environment. Dry powder inhalers are often less harmful to the environment but not always a clinically appropriate choice. By using secondary care data, researchers can now use the data to monitor the number of inhaler doses by product and calculate their potential CO2 equivalent.
We are able to match the information provided in the dataset to additional information available in the dm+d dictionary such as the ‘form’ of the VMP to allow us to report on items classed as ‘pressurised inhalation’ and ‘inhalation powder’ and compare dose for dose the volume issued.
The report does not account for clinical need of either form, rather a demonstration of the data available.
We can also use the guidance issued by NICE (https://www.nice.org.uk/news/article/nice-encourages-use-of-greener-asthma-inhalers) stating that metered dose inhalers have a carbon equivalent of 500g CO2 whereas dry powder inhalers have a CO2 equivalent of 20g, and apply this information to the provided data to get an estimate of the carbon dioxide equivalent of inhalers.
Summary
The release of Secondary Care data by the NHS will be useful to researchers within the healthcare and economy sectors but a clear understanding of the complex nature in which data is collated and processed is key.
If you would like more information about how Rx-info Ltd supports the NHS in its data needs please contact us at [email protected]
Footnote: Each virtual medicinal product in the SCMD contains a chemical, and this chemical was referenced against the WHOCC dictionary in order to structure each VMP in a reporting hierarchy and assign it a Defined Daily Dose (www.whocc.no). This means that large groups of similar chemicals or medicines can be pulled from the database into a report. The DDD allows a standardised value to be assigned to each medicine for utilisation reporting purposes.
Jason Pruchniewicz is a pharmacist at Rx-info Ltd with a background in NHS Clinical Pharmacy and a keen interest in technology and data.